[ad_1]
In a blog for Clinical Trials Day 2023, Derek C Stewart OBE, a non-executive director at the ISRCTN registry, discusses the importance of patient and public involvement in clinical trials

HRA #MakeItPublic logo
When health research is published, we tend to focus our minds on the findings and consider the implications for future treatments. Yet, what about those who took part in the trial? Too often they are forgotten and ignored.
In recent years there has been an increasing call to let trial participants know the results of trials in which they have taken part. The call has also been for this to be in a plain language summary.
As a strong advocate for more active patient and public involvement (PPI) in all aspects of health and care research following my own treatment for throat cancer, I welcome this positive development.
We have come a long way, despite the ravages that COVID-19 has left upon NHS services. Back in 1995 only one person in every 27 was on a trial in cancer. It rose to almost 1 in every 4. Health research gives us hope that things will be better for others in the future.

HRA logo
It seems fitting therefore to write about research transparency as we mark another International Clinical Trials Day on 22nd May – the anniversary of James Lind’s first recorded clinical trial in 1747.
The Health Research Authority (HRA) in the United Kingdom has been a key player in encouraging partners across the research system to work together to achieve greater transparency. I have had the pleasure of co-chairing the #MakeItPublic Campaign Group with Matthew Westmore, the HRA Chief Executive, encouraging collaboration.
The #MakeItPublic campaign has four strands: registering research studies, reporting results, informing participants, and sharing study data and tissue.
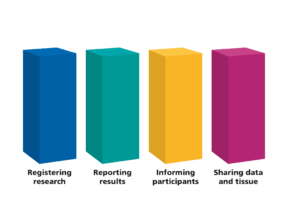
The four pillars of research transparency
This will make it easier for us all to find out about research that is taking place as well as help avoid duplication and waste. Hopefully, the frustrated cry of trial participants – “I took part in a study but never heard anything about it” – will be a thing of the past.
In December, I was delighted to join the ISRCTN board as a non-executive director. The ISRCTN registry has been a key influencer and enabler in providing key information about studies that are taking place.
The relationship between research and participant has changed in recent years. There is through the increasing PPI a shift to research being done ‘with’ people rather than just ‘to’ them.
Patients and the public are actively working beside researchers to ensure greater relevance to studies, better communication and more appropriate information. It is becoming more normal to see PPI on trial management committees and even assisting with trial methodology research. The language of co-design and co-production is increasingly in use.
#MakeItPublic circle infographic
This is demonstrated so clearly in the NIHR-funded INHALE study (registered at ISRCTN), which looked at whether using molecular diagnostics to diagnose hospital-acquired and ventilator-associated pneumonia in Intensive Care Units could promote better use of antibiotics and reduce antimicrobial resistance. The patient voice helped make the study more understandable.
This will be of vital importance when it comes to writing about the findings in plain language. Writing a lay summary for a protocol is challenging so it makes sense to involve and talk with patient communities about how to do this well.
The HRA Research Transparency Annual Report 2022-23 provides many other great examples of the cooperative work that is taking place.
I simply ask that we think more about the trial participant in all that we do. Without the willingness of members of the public to volunteer, attend additional consultations, give blood and tissue as well as donate money, we wouldn’t have the great research system we have.
[ad_2]
Source link